After treating hundreds of prostate cancer patients in Mexico, Bermuda and the Bahamas, Charlotte urologist Dr. Samuel Peretsman is planning his first HIFU procedure in Charlotte in early December. The technology for HIFU (high intensity focused ultrasound) was approved by the Food and Drug Administration in October. John D. Simmons jsimmons@charlotteobserver.com
For about 10 years, Dr. Samuel Peretsman has regularly traveled to Bermuda and the Bahamas to meet his Charlotte-area prostate cancer patients and treat them with a procedure that wasn’t approved in the United States.
But in October, the equipment used for HIFU – high intensity focused ultrasound – received approval from the U.S. Food and Drug Administration for treatment of prostate tissue.
So, after treating hundreds of patients outside this country, Peretsman will be one of the first to perform HIFU in the United States. The Charlotte urologist expects to treat his first patient on Dec. 11 at an outpatient surgery center in Huntersville.
HIFU (pronounced “high-foo”) is a noninvasive alternative to surgery and radiation for men who have early stage prostate cancer. Instead of cutting out the walnut-size prostate gland or destroying it with radiation, HIFU kills only the diseased tissue by cooking it with ultrasound waves.
Although HIFU was approved in more than 40 countries before the United States, some urologists continue to take a “wait and see” approach, noting that clinical trials comparing HIFU with other treatments have not been done in this country.
“I do think it will eventually find its place,” said Dr. Chris Teigland, a Carolinas HealthCare System urologist who has not performed HIFU. “This is just another tool. It’s going to take us awhile to figure out exactly where it fits in.”
Until October, HIFU was available in this country only through clinical trials for prostate cancer treatment. Elsewhere in the world, doctors also use it to treat pancreatic, breast, liver and kidney cancer.
Proponents of HIFU emphasize that it targets only the portion of the prostate with cancer, sparing healthy tissue and allowing most patients to avoid urinary incontinence and impotence, two of the dreaded side effects of surgery and radiation.
“I’m not affected by either of those. I’m one of the lucky guys,” said Howard Adams, 72, of Weddington. He had HIFU done by Peretsman at Doctors Hospital in Nassau, Bahamas, in July 2013.
Adams knows other men who have bladder-control problems and erectile dysfunction after prostate cancer treatment, and he wanted to avoid that. After speaking with other patients and doctors, he and his wife chose to pay $25,000 for HIFU, plus travel expenses. For now, HIFU is not covered by insurance.
“It was a big expense at the time, but I felt it was worth it,” said Adams, who is cancer-free but gets periodic checkups. “I’m happy for the guys today. Now that it’s been approved by the FDA, they can get the procedure done here.”
Charlotte origins
Charlotte businessman Steve Puckett Sr. founded the company, SonaCare Medical, that has been pushing for U.S. approval of HIFU since 2004.
The company, formerly known as U.S. HIFU, initially collaborated with an Indiana company that manufactured the HIFU device, called Sonablate. Puckett’s company eventually merged with the manufacturer and established HIFU clinics outside the United States. SonaCare Medical also paid for a U.S. clinical trial, hoping to demonstrate to the FDA that the procedure was effective at treating prostate cancer.
Results of that trial, involving 100 men who got HIFU for recurrences of prostate cancer after radiation, showed that 70 percent had no cancer after follow up biopsies. But last fall, an FDA advisory panel recommended against approval based on that data.
At the advice of the FDA, Puckett said his company then took a different, faster approach and sought approval of the device for “ablation of prostate tissue,” not specifically for treatment of prostate cancer.
The FDA approved that use in October. It means SonaCare Medical cannot market HIFU for cancer treatment, but surgeons can use it for that purpose.
“The urologist needs to make the decision based on their view of the patient’s condition and what they want to recommend,” Puckett said.
Only days after the FDA approved Sonablate on Oct. 9, another Charlotte company, HIFU Prostate Services, ordered five of the HIFU devices for installation in the United States. One was used for the first U.S. procedure on Nov. 10 in Louisville, Ky.
Another is slated for Huntersville, according to John Linn, a former executive with SonaCare Medical who founded HIFU Prostate Services this year. The local device is being installed Wednesday at an outpatient surgery center owned by a subsidiary of Carolina Urology Partners. That group of Charlotte urologists and Peretsman’s group, Urology Specialists of the Carolinas, are part owners of the local device.
As one of this country’s most experienced HIFU doctors, Peretsman is also part of a national group that will train and certify other U.S. doctors to use the technology. “Somebody has to go first,” Peretsman said. “I’m sort of grandfathered in.”
Less invasive treatment
Peretsman first learned about HIFU in 2005 when he saw a patient who had traveled to Germany for the procedure and needed a doctor in Charlotte. Soon, others followed.
“They would return to Charlotte and struggle to find someone to take care of them,” Peretsman said. “I thought if I’m going to start having to manage these patients, I may as well learn the technology. That way I’ll understand it better and know who’s appropriate for it and who isn’t.”
In the past nine years, he has traveled regularly, first to Mexico, and then to Bermuda and the Bahamas, to perform HIFU on Charlotte-area patients. The best candidates, Peretsman said, are men with “smaller, less aggressive” cancers that have not spread outside the prostate gland.
He compared HIFU to lumpectomy, the less invasive alternative to mastectomy (removal of an entire breast) for breast cancer treatment. Instead of removing the whole prostate gland – either with traditional surgery or robotic surgery – HIFU treats only the diseased section of prostate tissue, sparing nerves related to sexual and bladder function.
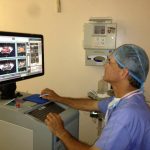
Peretsman doesn’t even touch his patients during most of the procedure. He sits at a computer where he controls a rectal probe that sends ultrasound waves to the patient’s prostate. Like a magnifying glass focuses light rays to a focal point, HIFU concentrates the ultrasound waves on a tiny area of diseased tissue and destroys it with high heat.
“It literally cooks it while you’re looking at it,” Peretsman said.
HIFU patients recover more quickly than surgical patients, who remain in the hospital for a day or two and take several weeks to recover. HIFU patients undergo general anesthesia, and the procedure lasts about two hours. But they can go home the same day, and they are monitored to make sure they stay cancer-free.
Cautions about HIFU
Doctors who have reservations about HIFU stress the FDA did not approve the procedure specifically for treatment of prostate cancer. They say questions remain about which patients are appropriate for HIFU and what outcomes will be over the long term.
HIFU is recommended for patients with low- to medium-risk prostate cancer. Some of those patients can live for decades without their cancer progressing. As many doctors say, these are patients who may die with prostate cancer, not of prostate cancer.
That raises the question of whether they should get any treatment, said Teigland, the Carolinas HealthCare urologist. “The big trend right now is to watch these patients and not treat them at all, because they progress so slowly. The question is, ‘Do these cancers need to be cured?’ ”
Instead of treating low-risk prostate cancer patients, Teigland said doctors are increasingly monitoring them closely, a practice called “active surveillance.” He said about a quarter of those patients end up getting treated because the cancers progress.
Teigland added that patients who choose HIFU should make sure they find surgeons who have experience doing the procedure. Just like performing surgery with the daVinci robot, using the HIFU device requires expertise, he said. “You can burn the rectum. You can burn the bladder. It’s just a machine. It needs a good pilot.”
Dr. Behfar Ehdaie, a prostate cancer specialist at Memorial Sloan Kettering Cancer Center in New York, said it’s important for patients to hear all their options. He said HIFU should be performed only by skilled surgeons who enroll patients in a clinical trial or registry that provides long-term monitoring.
Sloan Kettering is conducting a clinical trial that could help determine which patients are good candidates for HIFU, he said. “We’re still determining the right patients to treat.”
Based on data about prostate cancer patients treated over the past 10 years, Ehdaie said, “we believe a majority of these men were overtreated. … It doesn’t mean they didn’t have cancer. It just means that immediate treatment may not be in their best interest.”
Reaction to critics
As one of the most experienced HIFU users in the country, Charlotte’s Peretsman agrees with Teigland and Ehdaie that patients should choose doctors who have mastered the technique.
Patients who have the HIFU procedure in Huntersville will be enrolled in a registry for long-term followup, he said. But he added that there was never a registry for all patients treated with robotic surgery, radiation or cryoablation, a treatment that uses extreme cold to destroy prostate tissue.
Peretsman offers all of those therapies and said he never tells patients that HIFU is their best option. He said it “fits some patient expectations better” if they are concerned about maintaining sexual function and continence, and if they understand that followup testing is necessary to detect a recurrence that would require additional treatment.
Finally, he said other devices, such as the daVinci robot, never received FDA approval specifically for prostate cancer either. “Yet we have used them, and continue to use them, and will continue to use them.”



Comments are closed.